There has been a fair amount of interest in fish oil as an antiinflammatory medicine over the years. In part this is driven by fish oil being “natural”. The scientific reason is that oils in the diet are the building blocks for certain chemicals the body makes, some of which are immune modulators. Fish oils contains a group of oils known as omega-3 fatty acids (chemically a double bond is present 3 down from the end of the carbon chain of the fat molecule). These oils are able to prevent the production of certain inflammatory molecules in the human body.
Fish oil per se is not a defined substance. There is the matter of variation of the fish, whether the oils is taken from whole fish sqeezings or just the fillets, and what processing is done. The whole fish issue is important because toxins (or perhaps useful drugs) are concentrated in fish livers. Processing is important because of stability. As with all supplements marketed as foods, the natural food pushers do not want regulation to be exerted upon them as it would add all sorts of complexities to their lives. As I see it, if they are going to push their own products, the consumer should be assured that the manufacturer can trace the source of raw material, have a regular way of making the stuff, keeps records of quality control, and does assays of the important ingredient(s). Additionally, the studies, if any, used to support the use of natural products often are used to recommend products different from those in the study. With something as unregulated, unassayed, and undefined as health food store products, who knows whether the health food store drug will do any good.
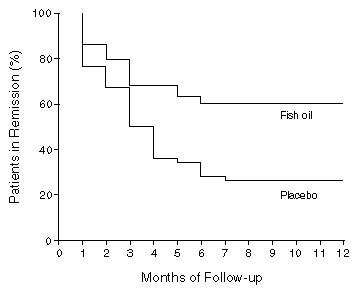
With this background, now comes a drug which is a fish oil preparation. Researchers in Italy used a fish oil preparation called Purepa, manufactured in Sweden, and coated the capsules with an enteric coating called Eudragit NE 30D. It is well defined and manufactured with the care a drug company takes. A study in the New England Journal of Medicine (NEJM 334(24):1557-1560, 13 June 1996) reported that patients with Crohn’s disease had less relapses when taking the medicine than did those taking placebo. The study was well done. It used a group of patients randomly assigned to either drug or placebo. Patients were not able to tell which drug they were on. (Which also implies that enteric coated Purepa does not have the nasty fish taste which can make fish oil hard to take.) The patients who were asked to participate were known to have a high chance of flares of the disease, based on their having all been in remission less than 2 years, with serum alpha 1-acid glycoprotein concentration > 130, alpha 2-globulin > 0.9, or a sed rate > 40. The findings were remarkable. The patients on drug had a relapse rate of only 41% compared to controls who had a relapse rate of 74% over the one year of followup. Diarrhea was a side effect in 4 of 39 fish oil treated patients vs 1 of 39 placebo patients. The diarrhea did not resolve when the drug was stopped in experimental or control subjects. The dosage of fish oil was 9×500 mg capsules per day, = 4.5 grams of fish oil per day. Interestingly, all relapses occurred within 7 months in both groups. Also, the fish oil group had improvement in all three serum measurements made, while the placebo group all had worsening of the measures.
The problems with the study concern whether the drug will be effective in all patients with Crohn’s, or only those patients like those in the study. This is a real issue – Crohn’s disease is a heterogeneous group, and the Italian group that published this may have selected a subset of Crohn’s that would respond differently. Also, this study was done in Italy. The Italian diet differs from a typical American diet, not to mention Scottish, New Zealand, Chinese, Dutch, Polish, German, French, or any cuisine that you may wish to identify. Italians also are heterogeneous in their diet, varying greatly from North to South, and inland vs seacoast. The study did not subgroup its results by region of Italy where subjects were born or lived, which would have been interesting.
It looks like this will be effective, however. Given the safety of the preparation it will be a welcome addition to treatment of Crohn’s. The variation in fish oil preparations available, along with the history of previous negative studies with fish oils, leads me to conclude that until a particular preparation is studied it will not be possible to say whether any particular fish oil preparation will work in Crohn’s. It is interesting that both placebo group and Fish Oil group had no more relapses after 7 months. It would be nice to know if the changes in any of the blood measures predicted who would relapse. The relapse curves also raise the question whether patients only need to be on the drug for 7 months to get the full benefit.
Stephen Holland, M.D.
University of Illinois College of Medicine at Urbana-Champaign