Readers can get access to the New England Journal of Medicine (NEJM) where 3 articles can be viewed every month. This requires a simple signup. This is such a useful tool I added a navigation page about it.
Month: July 2019
IBD Sucks/QurlyJoe site added to links
The QurlyJoe site, also known as the IBD Sucks site, is added to the Links page
The site IBDSucks was added to the links.
The website IBD Sucks is aptly named. It is an older site, but has a very focussed set of links related to IBD. Back in the day, it was known as the QurlyJoe site. I still don’t know what that means. Now, there are chat rooms there for support. A simple registration is needed, but get inside and lots of links!
Anti-TNF drugs in ulcerative colitis may not last forever
The duration of benefit of Anti-TNF drugs for ulcerative colitis is not well understood, This article shows about 3 years of benefit on average with some UC patients getting 7 years or more of a response.
When Remicade came out it was a breakthrough in treatment for patients with Crohn’s disease. Eventually it came to be recognized as a treatment for Ulcerative Colitis as well. We went through a time of using it while on mesalamine and azathioprine, then just Remicade alone, now back to with azathioprine at low dose, and perhaps now with mesalamine again.
Aside from how to best administer anti-TNF drugs it was found that patient’s immune systems could make antibodies against them. After all, the drugs like Remicade (infliximab) and Humira (adalimumab) are themselves antibodies and therefor are proteins. The patients’ immune systems see these as foreign proteins and therefor can develop an immune reaction against them. Infliximab and adalimumab were designed to minimize immugenicity. (Immunogenicity is the word that describes no stimulating something is to the immune system). However, the immune system responds very well to foreign substances. An immune reaction often develops.
Inflammatory bowel diseases are also the result of many aspects of the immune system driving a reaction. While Tumor Necrosis Factor is one of the control proteins of the immune system it is not the only one. A patient may have multiple drivers of their disease in addition to TNF. This probably explains why only about half of patients respond to Remicade in the first place.
Once a patient responds and is on infliximab or adalimumab will it work forever? Gastroenterologists find that after a few years patients may stop responding. Tests can be done to see if antibodies are forming or if the concentration of the drug is low. But sometimes the effectiveness of anti-TNF drugs just wanes.
Crohn’s patients have been treated the longest, since the anti-TNF drugs were first used in them. We see very long duration benefits of anti-TNF drugs in many patients with Crohn’s. A number of studies have looked at the time course of response in Crohn’s disease.
Ulcerative colitis has been treated with anti-TNF drugs for a lesser number of years, so there are fewer articles obout the effectiveness of anti-TNF drugs in ulcerative colitis. An article in IBD Journal, Treatment Persistence of Infliximab versus adalimumab in Ulcerative colitis: a 16 year single-center experience[1], addresses the issue.
In this study researchers at the Nancy University Hospital in in Nancy, France reported on the results of treatment of ulcerative colitis patients that had been on long term therapy for at least 6 months. They selected patients who were treated for UC with either infliximab or adalimumab. Patients treated just for pouchitis were not included and neither were patients who had just gotten intermittent doses.
They found 160 UC patients that fit the definition of chronic use of and anti-TNF for over 6 months. In that group 43 patients had started on one of infliximab or adalimumab and swithced to the other. On average, patients responded for about 3 years (3.1 years for infliximab and 2.1 years for adamimumab. Patients that were also on mesalamine drugs had a longer duration of response.
The telling graphic from the study is the analysis of time to failure of anti-TNF treatment, figure 2 in the article.
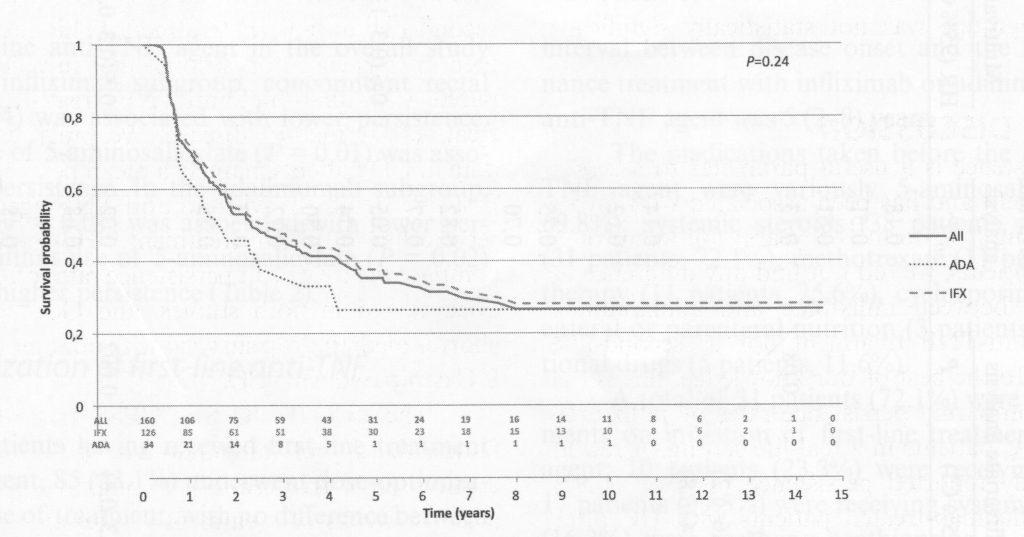
The label on the vertical axis “Survival Probability” means the proportion of patients for whom the anti-TNF drug continued to work, not time to death. The label could better have been “Proportion of patients still in remissiokn.” The number at the top is 1, meaning 100%.
Looking at the graph everyone was good for a few months, but then the drop-off is evident. While the average time of benefit was about 3 years, that includes the people with long term benefit. So for infliximab about half of individuals lost benefit at 2.3 years, and about 25% of patients had benefit that exceeded 10 years. For adalimumab, about half of individuals lost benefit at 1.7 years, but, similar to infliximab, about 25% had long term benefit.
This sort of data is very helpful in deciding on how to use anti-TNF medications. The study only looks at infliximab and adalimumab. Golimumab, certolizumab were not included, but those drugs are not as commonly used as infliximab and adalimumab in the treatment of UC.
The study is from an academic medical center, where patients are referred from the community, often sicker and less responsive than patients in the community. Thus, results may be worse than seen in community centers.
Reference:
1. Lieven Pouillon, Cédric Baumann, Hélène Rousseau, Myriam Choukour, Charlotte Andrianjafy, Silvio Danese, Laurent Peyrin-Biroulet, Treatment Persistence of Infliximab Versus Adalimumab in Ulcerative Colitis: A 16-Year Single-Center Experience, Inflammatory Bowel Diseases, Volume 25, Issue 5, May 2019, Pages 945–954, https://doi.org/10.1093/ibd/izy322
A review of new drugs for treatment of IBD
Perhaps you’re getting confused by all the medications that are now available for treating IBD. A recent article in IBD discusses the drugs by groups.
Year ago all we had to treat IBD was prednisone. Sulfasalazine ushered in the era of mesalamine drugs. Azathioprine was available as well. Then it got interesting. Our colleagues in immunology research looked into what drove the immune response and identified cachexin, also known as Tumor Necrosis Factor alpha, or TNF-α for short, as a major signaling protein in immune responses. cA2 was an antibody directed against TNF-α, which was shown to be useful in Crohn’s. Soon named Infliximab, the antibody revolutionized treatment of inflammatory bowel disease.
But our immunology colleagues have not been idle. The immune response control system has ben studied for years now, and additional targets ahve been identified in the immune system. We now have more drugs than we could have dreamed of years ago for the treatment of IBD.
A recent review [1] published in IBD Inflammatory Bowel Diseases, a journal of the Crohn’s nd Colitis foundation has provided a nice summary of some current and new drugs for use in IBD. It’s worth a read. I’m providing some highlights here.
First off, the graphic on the cover of IBD is awesome. The immune system is complex, and a cartoon such as was on the cover is very helpful in putting a model in the readers head. I took this diagram off the cover of the issue of IBD. I really like the diagram. You can see that it shows a number of the molecules that are involved in the immune system of IBD.
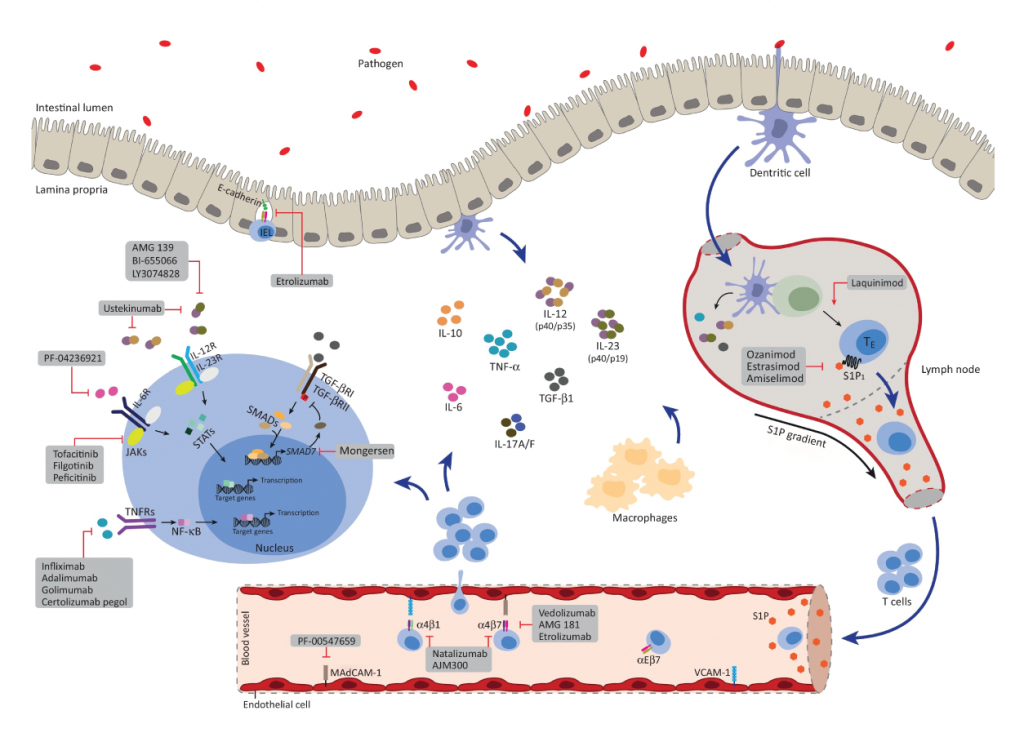
Anti-adhesion agents
First mentioned in the review are the Anti-adhesion agents. You see, for the immune system to deliver immune cells to that are of inflammation, the cells of the immune system have to migrate to the area on inflammation. I recall stunning videos of immune cells whipping around in the vascular system, with cells stiopping in surreal instantaneity at the place they need to migrate, and then migrate through the wall of the blood vessel to the are of inflammation. If you can stop cells from finding where they need to go, the immune cells will not get to their targets and an inflammatory resposnse can be prevented. Natalizumab was the first drug in category to come to clinical practice. A protein on the surface of immune cels, α4β7/α4β1, binds to a structure named MAdCAM-1 and a structure on the inner surface of blood vessels (VCAM-1 – Vascular Cell Adhesion Molecule-1). Natalizumab is an antobody that binds to α4β7/α4β1, shich bloks cells from binding to blood vessel linings, thus preventing an immune response.
Regretably, natalizumab has been associated with the develoopment of Progressive Multifocal Leucoencephalopathy, a dangerouse disease caused by reactivation of infection by the JC virus, a not uncommon infection that the immune system usually keeps under control.
This is an example of how, even though we have a promising new drug, surprises appear when we block the immune system, allowing an infection that stays controlled by the immune system.
Vedolizumab is another anti adhesion drug. This antobody just blocks the α4β7 protein. Progressive Multifocal Leucoencephalopathy does not seem to occur with this drug. The review nicely describes the studies that support the use of Vedolizumab.
The review describes a drug under study, Etrolizumab, which is directed against the β7 molecule, so it is effective in interfering with several of the proteins that are involved with cell binding to surfaces.
AJM300 is another drug under study, but is special because it is not an antibody. It is a small molecule which blocks immune cell binding to the MAdCAM-1 and VCAM-1 proteins. This is a welcome new agent, as it can be taken as a pill rather than an injection, which is needed by antibodies. Time will tell what adverse reactions happen. Certainly, the incidence of progressive multifocal leucoencephalopathy will need to be watched for, since that problem was seen with natalizumab.
Anti-interleukin Inhibitors
Aside from TNF-α, other immune proteins are involved in the cascade of immune reactions that drive inflammatory bowel disease. IL-2, IL-12, IL-23 are all important. While prednisone blocks IL-2, the drug ustekinumab blocks IL12 and IL-20. It is effective in Crohn’s disease and psoriasis.
Risankizubmab is under development. It looks like it will also be effective in Crohn’s disease.
JAK/STAT inhibitors
In the immune system a number of chemicals are important in regulation of the immune system besides the interleukins. That is because the proteins that regulate the immune system interact with cells which then elaborate internal chemicals in response to stimulation by the interleukins. One such system is the JAK/STAT proteins. If those cellular signalling systems can be blocked then the immune system can be shut down in another way.
Tofacitinib is currently on the market, and works by blocking the JAK system. It works in Psoriasis and Ulcerative Colitis. It is a small molecule, and taken as a pill rather than an injection.
Filgotinib also blocks the JAK system, but works better in Crohn’s disease. It is currently under investigation.
Spingosine-1-phosphate receptor modulators
The review gives short shrift to this class of immun modulators, and honestly, I need to brush up on this system to tell more about what it does in the body. A drug, Ozamimodh is a small moledule orally administered It is in development and is promising for Ulcerative Colitis.
Stem Cell Therapy for perianal Crohn’s Disease
The review describes the injection of stem cells for treatment of perianal fistulas. As the review states, it isn’t known how stem cells work. These are very experimental treatments. The therapy advertised which uses cells from the stems of plants are not stem cells in the sense of stem cells from humans and have nothing to do with this.
Conclusion
This review looks over the new therapies of Anti-adhesion agents, Anti-interleukin Inhibitors, JAK/STAT inhibitors, Spingosine-1-phosphate receptor modulators, and Stem Cell Therapy. While it is written fo rthe professional, a non-professional can glean the complexity of the immune system and the exciting new options for treatment that the future protends. I’d quibble with the term Anti-interleukin Inhibitors since Anti-interleukin antibodies would be a better term. But overall the paper does describe new treatments that are available and others that will be available in the future.